Michael Rapaport Quote: “The effect hip-hop had on me was enormous. I was exposed to it by happenstance. My father worked at a radio station in N...”

Mar. 1, 2001 - 31428JBB: DENISE RICH AND NATALIE COLE TO ACCEPT.AWARD FROM WKTU RADIO STATION . AT CLUB EXIT, NYC. PERFORMANCE BY SHAGGY 03/01/2001. JOHN BARRETT/ 2001(Credit Image: © Globe Photos/ZUMAPRESS.com

New York Radio Personality “Paco” of Disco 92 WKTU Has Died - Urban Radio Nation | R&B Radio, Hip Hop Culture, Black Media Personalities, and Sports Media

Singer Ashanti reacts to photographers backstage of the "Miracle on 34th Street" concert hosted by radio station WKTU in New York City, December 18, 2002. REUTERS/Jeff Christensen JC Stock Photo - Alamy









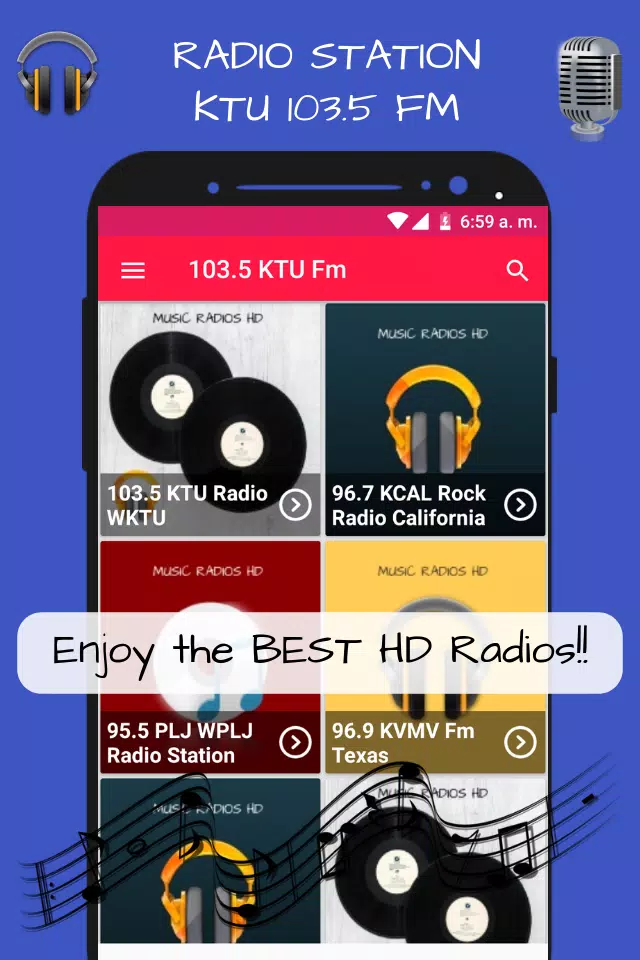
![▷ [Listen to] WKTU - KTU 103.5 FM ← Free RADIO station 2023 live and online ▷ [Listen to] WKTU - KTU 103.5 FM ← Free RADIO station 2023 live and online](https://escuchar.radio/assets/images/radios/z100ny.jpg)