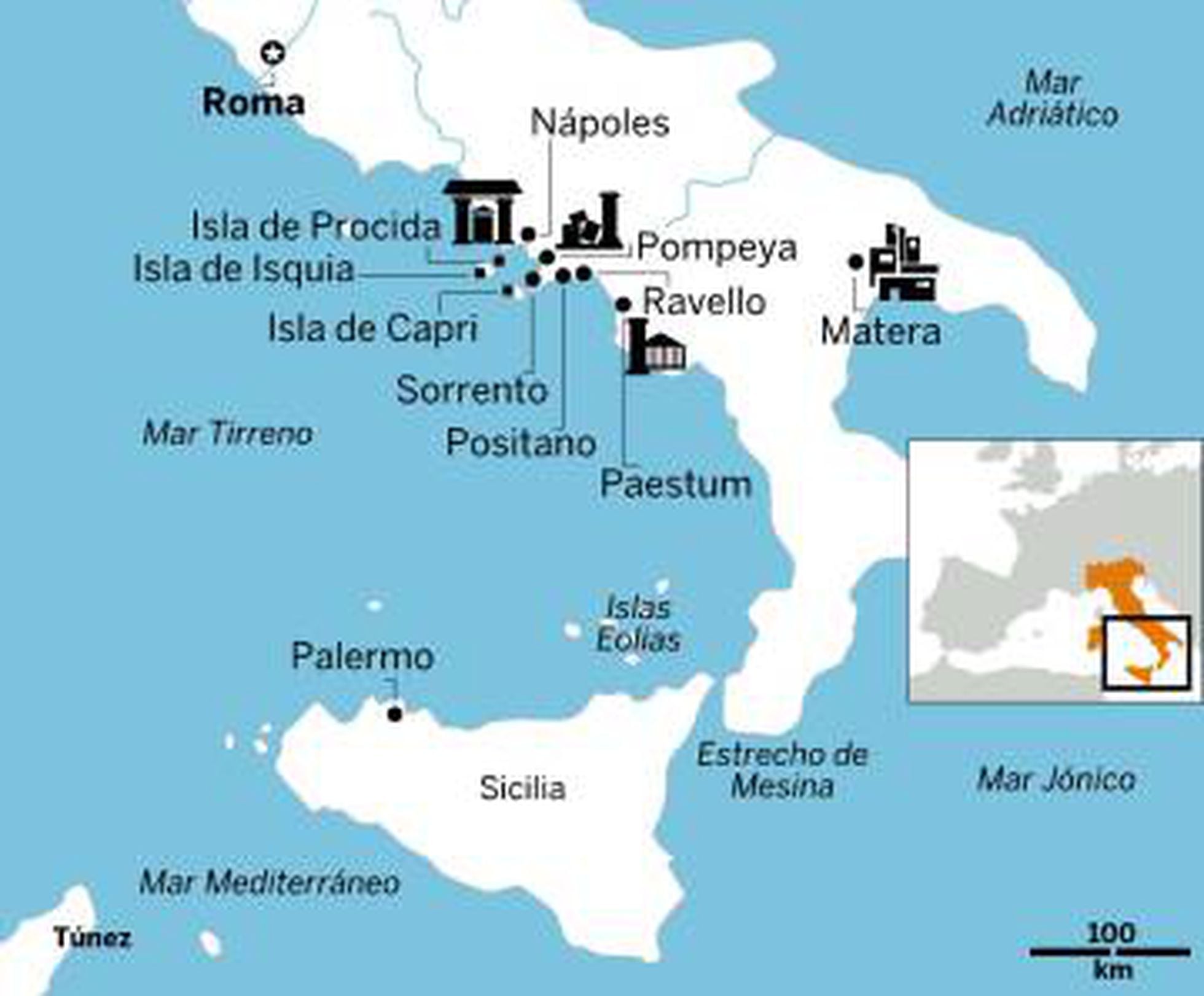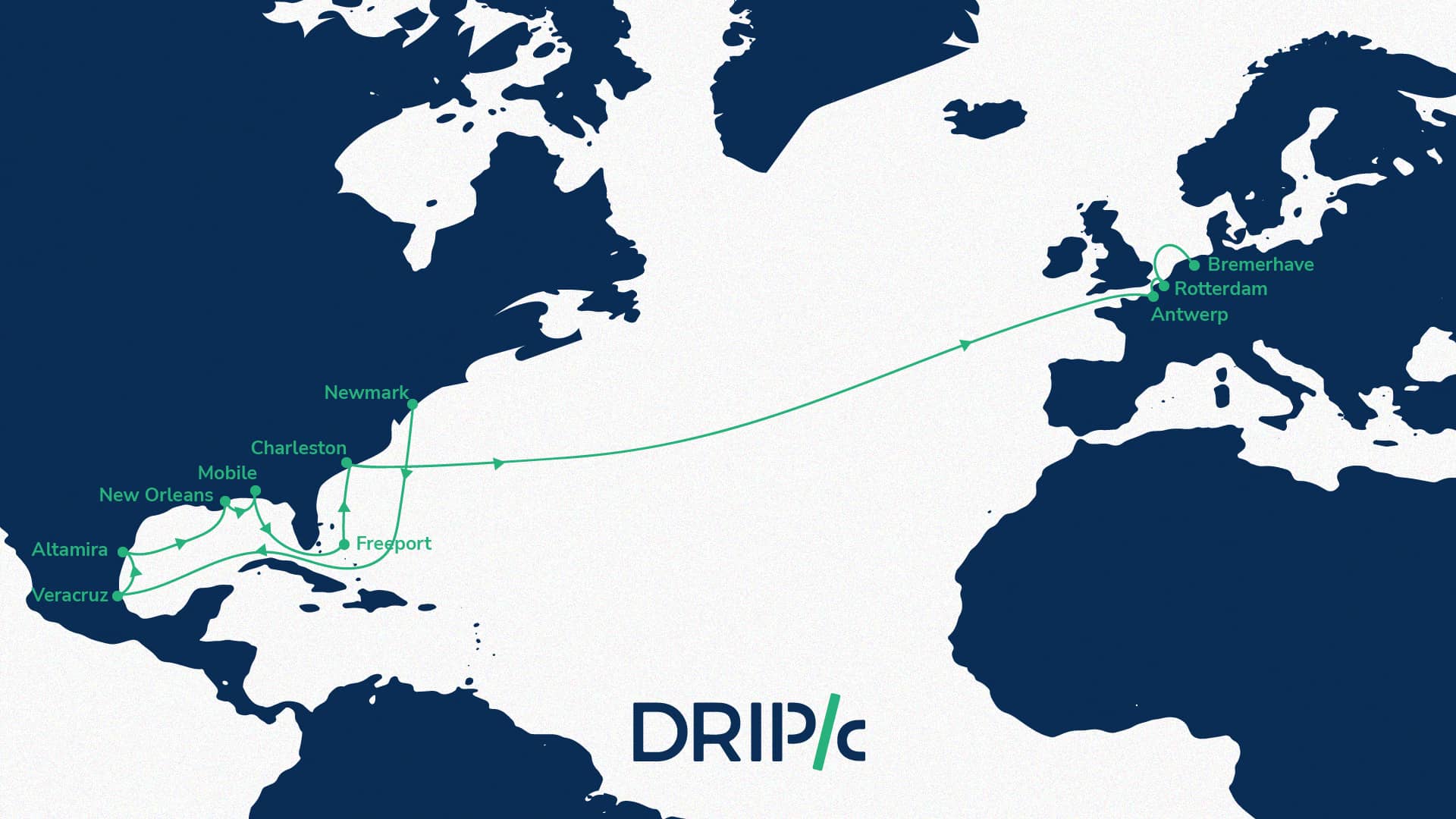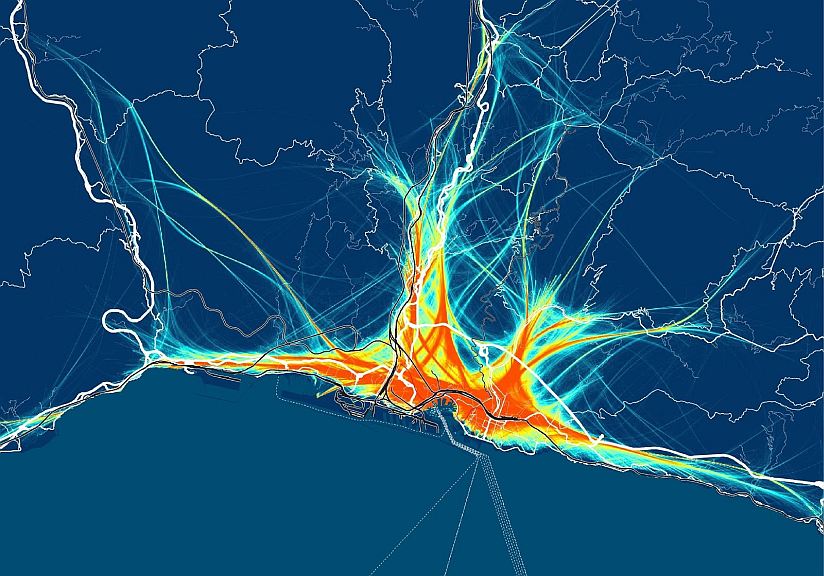
La naturaleza multiescalar de la movilidad portuaria, más allá del umbral de puerto-ciudad. El caso de dos ciudades portuarias del norte de Italia: Génova y Venecia - AIVP
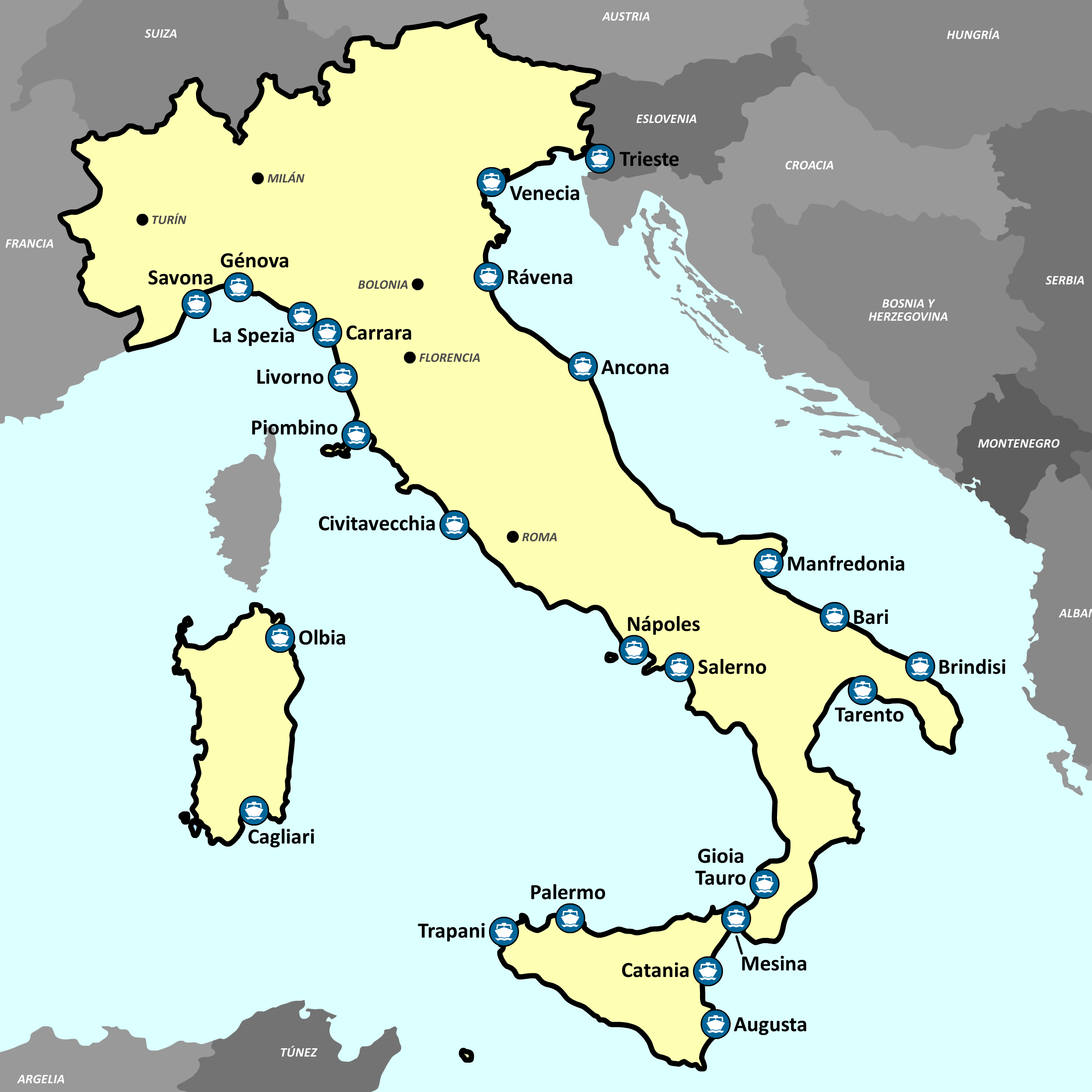
Mapa político y administrativo a gran escala de Italia, con alivio, carreteras, ciudades, puertos marítimos, aeropuertos y otras marcas | Italia | Europa | Mapas del Mundo