
Amazon.com: Inateck Surface Pro 7 Keyboard, Bluetooth 5.0, 7-Color Backlight, Compatible with Surface Pro 7/7+/6/5/4, KB02026 Gray : Electronics

Microsoft Signature Keyboard/Cover Case for 13" Microsoft Surface Pro 8, Surface Pro X Tablet - Black - Walmart.com

Brydge new hard keyboard for the Surface Pro 8 turns the tablet into a clamshell laptop - MSPoweruser

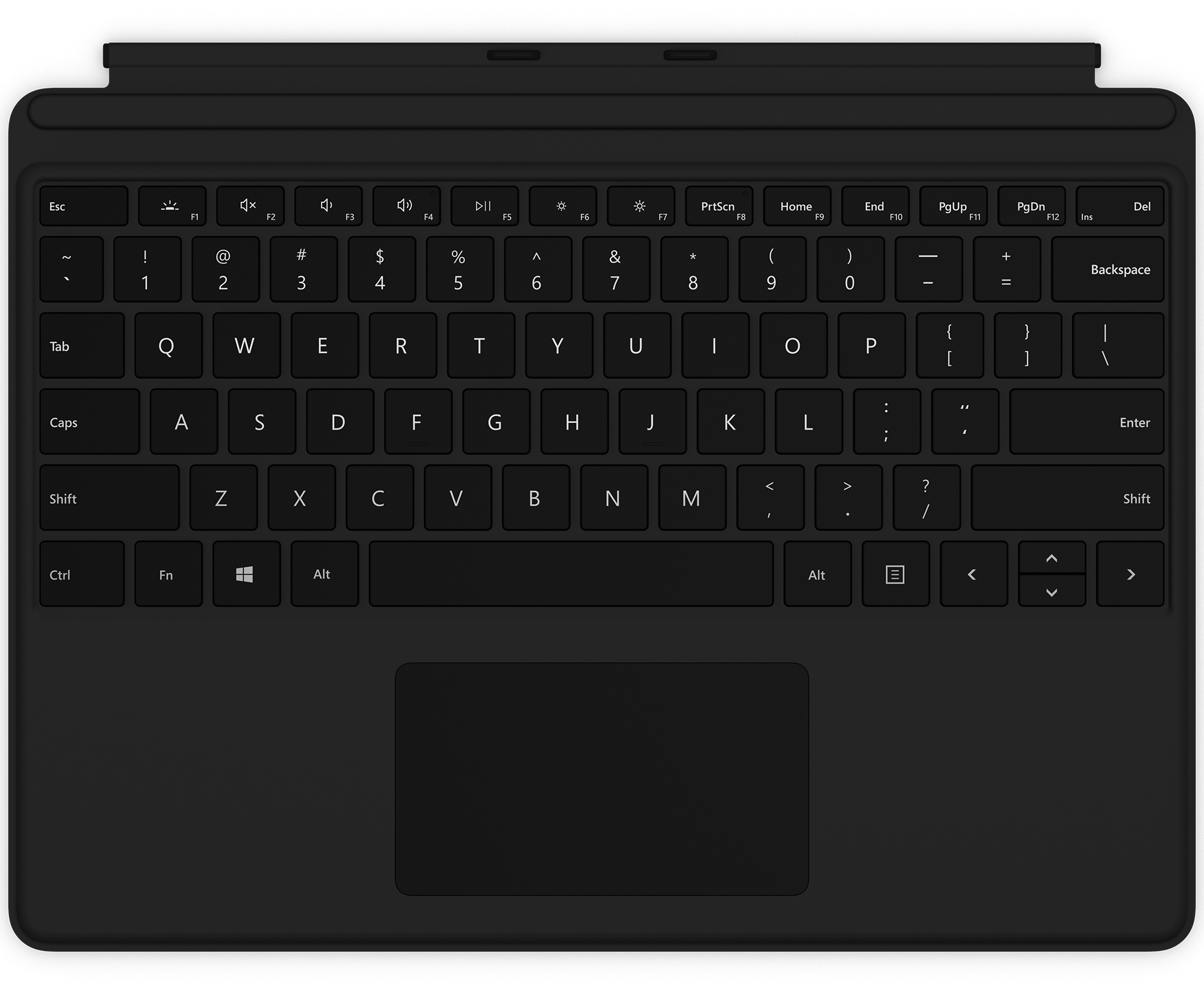
Brydge SP+ review for Surface Pro 8: A fantastic way to get a full laptop for just $100 | Windows Central

Bluetooth Microsoft Surface Pro 7/Pro 6/Surface Pro 5 (Pro 2017)/Pro 4 12.3 inch Tablet/Surface Pro 3 2014 Keyboard Case with Trackpad - Detachable Wireless Keyboard Type Cover - Walmart.com

Microsoft Surface Pro Signature Keyboard for Pro X, Pro 8 and Pro 9 with Surface Slim Pen 2 Forest 8X6-00121 - Best Buy

Amazon.com: Surface Pro 7 Wireless Bluetooth Keyboard with Touchpad 7 Color Backlit Rechargeable Battery Detachable Keyboard for Microsoft Surface Pro 4/5/6/7/7+ : Electronics

Amazon.com: GreenLaw Surface Pro 9 Keyboard, Pro 8, Pro X 13 inch, Multi-Gesture Touchpad, 7 Color Backlight, Wireless Bluetooth 5.1, Detachable Ultra-Slim Type Cover for Surface Pro 13 inch, Grey : Electronics















/cdn.vox-cdn.com/uploads/chorus_asset/file/22869358/unnamed.jpg)