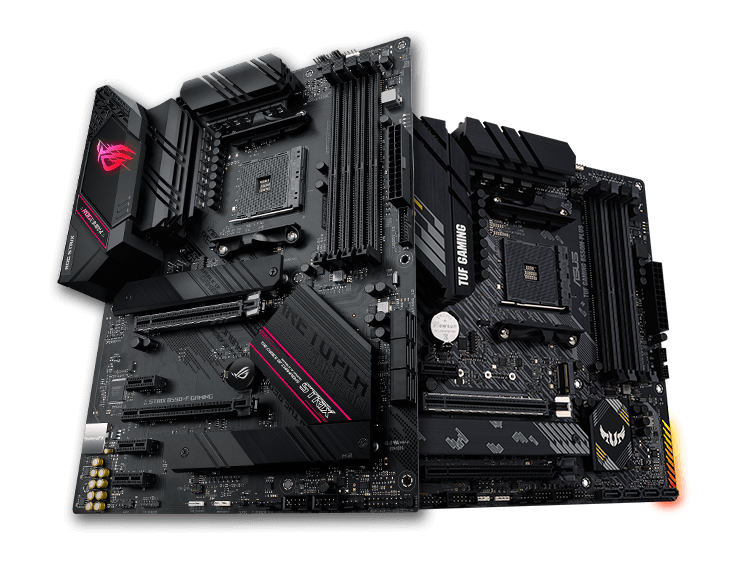
ASUS Carte Mère Gaming ROG Crosshair VIII Hero (Wi-Fi) AMD AM4 Ryzen 3000 (16 power stages PCIe 4.0 M.2 DDR4 OptiMem III Wi-Fi - Cdiscount Informatique

Kit Upgrade PC AMD Ryzen 5 3600 MSI X570-A PRO 16 Go - Kit upgrade PC - Garantie 3 ans LDLC | Muséericorde

Gigabyte X570 Gaming X (AMD Ryzen 3000/X570/ATX/PCIe4.0/DDR4/USB3.1/Realtek ALC887/HDMI 2.0B/RGB Fusion 2.0/Realtek GbE 8118 LAN/carte mère de jeu) - Walmart.ca

Ryzen 3000 : AMD propose bien un kit de démarrage pour mettre à jour sa carte mère en dernier recours - Les Numériques

Gigabyte X570 Gaming X (AMD Ryzen 3000/X570/ATX/PCIe4.0/DDR4/USB3.1/Realtek ALC887/HDMI 2.0B/RGB Fusion 2.0/Realtek GbE 8118 LAN/Carte mère de jeu) : Amazon.ca: Électronique

B550 AM4 mini-itx carte mère B550SD4-ITX pour AMD Ryzen 3000/5000 processeurs de la série DDR4 touristes Channel - AliExpress

Carte mère socket AM4 pour ordinateur de bureau, CPU série Ryzen 3000 5000, AMD B550, TUF GAMING Bcape M PLUS WIFI II AM4 DDR4, 128 Go - AliExpress

Carte mère B550 pour AMD Ryzen 3000/5000, cpu MSI MPG B550 GAMING PLUS, AM4, PCI-E 4.0, usb 3.2, M.2, ATX - AliExpress

ASUS Carte Mère Gaming TUF Gaming X570-Plus (Wi-Fi) AMD AM4 Ryzen 3000 (PCIe 4.0 M.2 12+2 Dr. MOS DDR4 LAN HDMI DP CFX USB 3.2 Gen 2 Type-A Type-C Aura Sync RGB

ASUS Carte Mère Gaming Prime X570-P AMD AM4 Ryzen 3000 (12 DrMOS power stages PCIe 4.0 M.2 DDR4 HDMI CFX SATA USB 3.2 Gen 2 Aura - Cdiscount Informatique