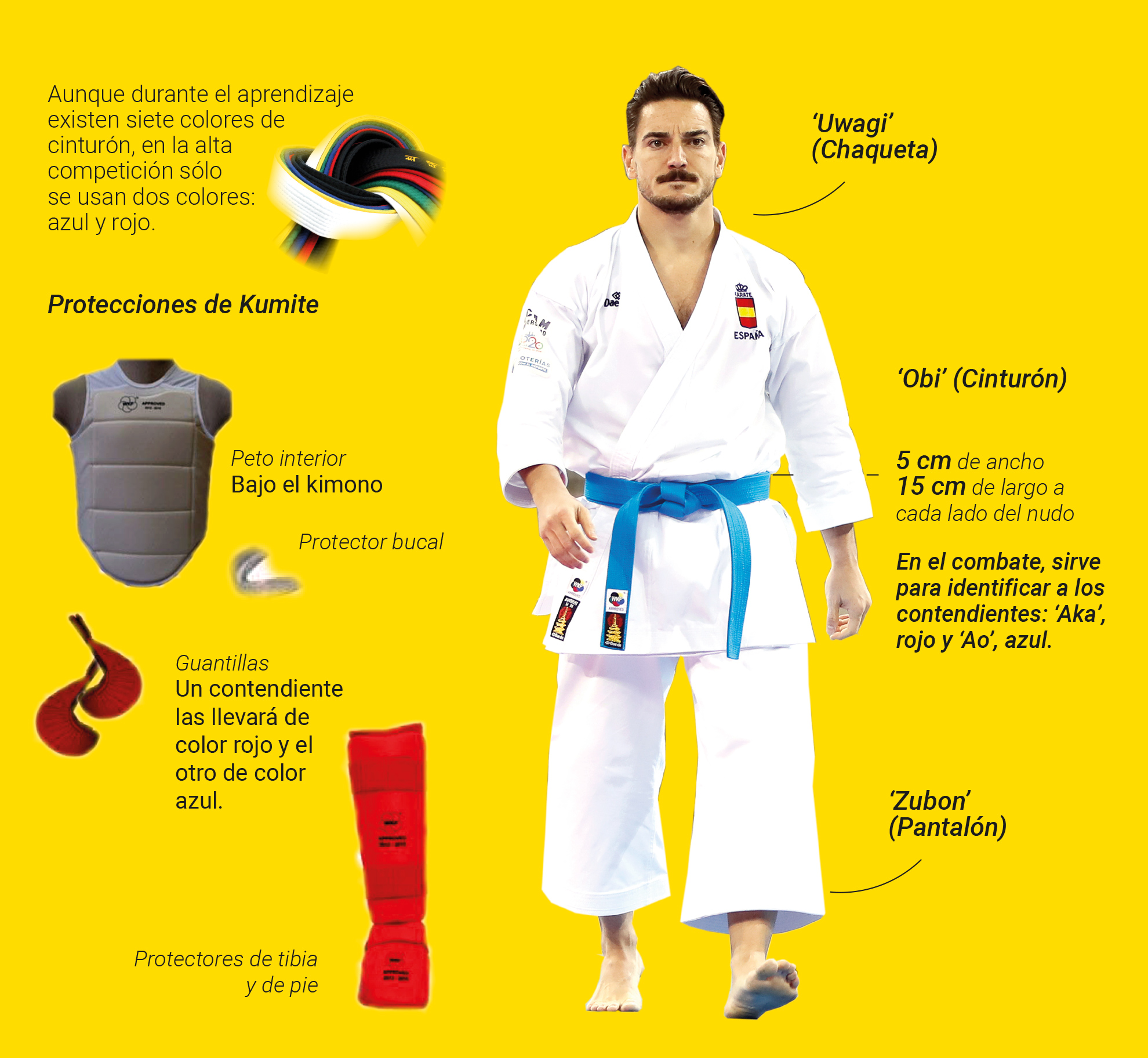
Amazon.com: Karate Do Kimono Cinturón Amarillo Lobo Haciendo Cool Kata Move Sudadera, Negro - : Ropa, Zapatos y Joyería
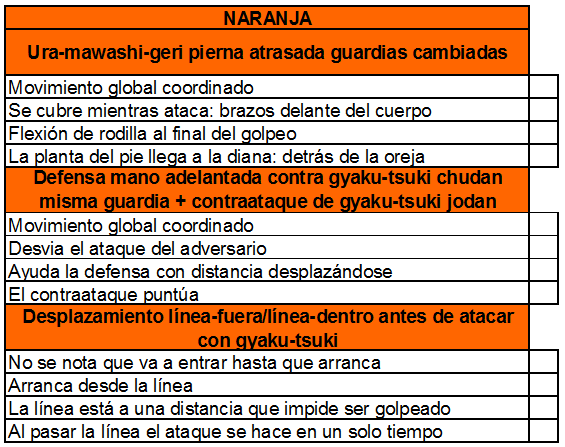
planilla de examen para los cinturones amarillo-naranja y naranja. Se... | Download Scientific Diagram

planilla de examen para los cinturones blanco-amarillo y amarillo. En... | Download Scientific Diagram