
Moulinex Cone Holder Cutting Cubes Fresh Express Dj750 Dj753 Dj755 Dj800 Hv4 - Food Processor Parts - AliExpress

Grater Moulinex Fresh Express Red/white 150 W (refurbished C) - Fruit & Vegetable Tools - AliExpress

Moulinex DJ756G Fresh Express Plus electric grater Red, White - Meat grinders - Kitchen appliances and accessories - Home appliances - MT Shop
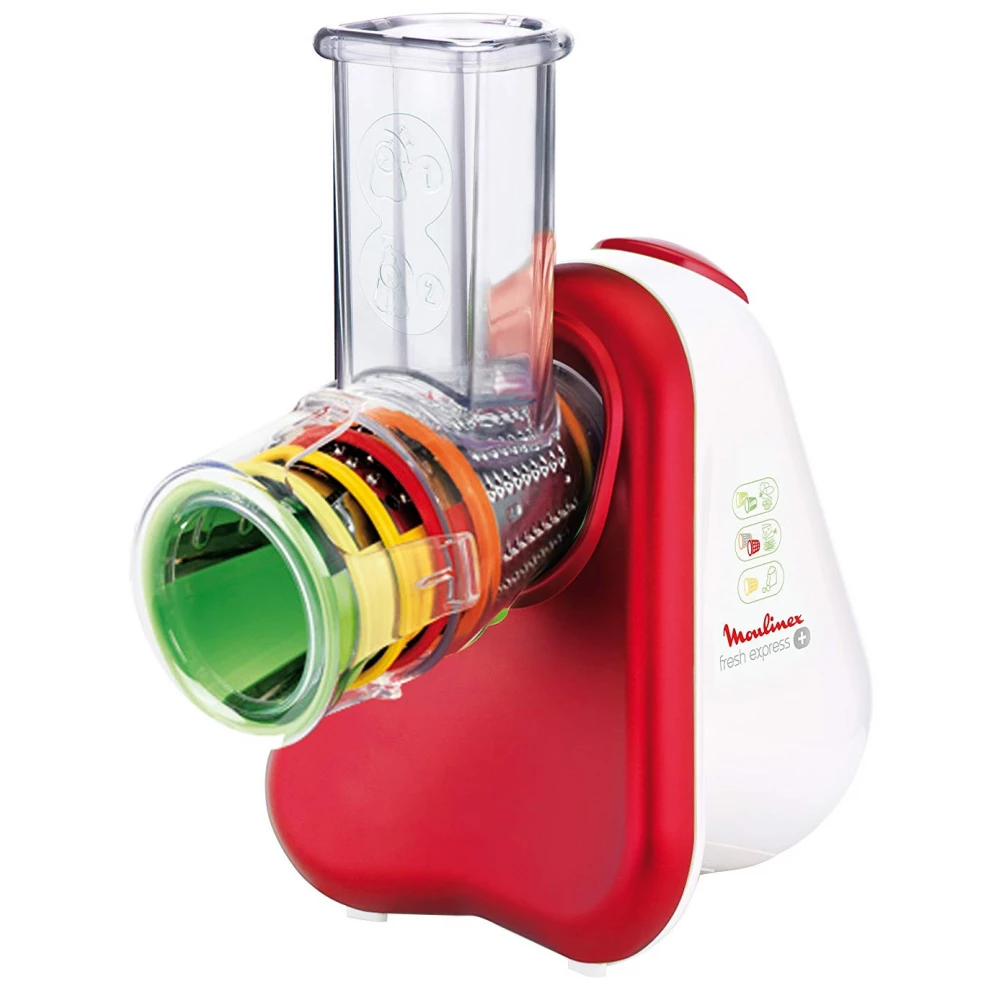
MOULINEX DJ756G Fresh Express Plus Electronic file red / white - iPon - hardware and software news, reviews, webshop, forum

MOULINEX DJ5005 Fresh Express Move shredder - iPon - hardware and software news, reviews, webshop, forum















